5 Considerations for Developing an Innovative mHealth Strategy
 With mHealth touted as the next big thing in the health information technology space, med tech companies should be “thorough and deliberate” when determining an mHealth strategy, says a new report from Deloitte.
With mHealth touted as the next big thing in the health information technology space, med tech companies should be “thorough and deliberate” when determining an mHealth strategy, says a new report from Deloitte.
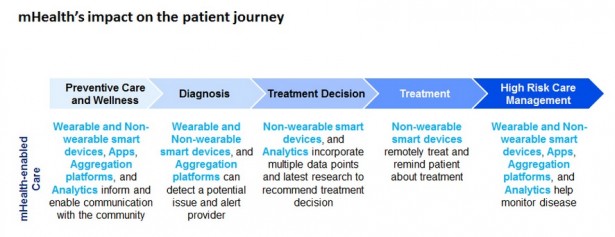
The report, “Mobilizing MedTech for mHealth,” defines critical decision points for med tech companies designing and deploying mHealth-enabled care, including preventative care and wellness, diagnosis, treatment decision, treatment itself and high-risk care management.
And there’s plenty of value to go around, as the global mHealth market was evaluated at $10.5 billion in 2014 and is expected to grow 33.5% annually between 2015 and 2020.
mHealth solutions span applications (apps), smart devices (wearable and non-wearable), aggregation platforms and analytics, creating business models across Deloitte’s “information value loop,” which describes how information creates value, how to increase that value and how understanding the relevant technology is central to positioning an organization to capture value.
To capitalize on potential opportunities in mHealth, Deloitte recommends considering the following for your mHealth strategy, for growth and sustainable advantages:
- Make a strategic decision on whether to embrace mHealthand whether this will be driven at the corporate, business unit, or product line level. Med tech companies should identify where in the information value loop it is most likely to monetize an mHealth solution.
- Target the diseasesfor which the organization wants to leverage mHealth solutions and identify how these solutions can provide value in the patient journey through data and results from studies that show these solutions work in real populations to help change behavior, engage patients, and improve outcomes.
- Collaborate with other organizationsaligned on a similar patient experience vision. This can provide a mechanism to bring mHealth solutions to patients while leveraging innovation, technical competencies, and commercial and regulatory expertise.
- Expand the scope of regulatory awareness beyond the FDA.Multi-agency oversight is likely to become the new norm as the FTC, FCC, OCR and others become more involved in the creation and enforcement of mHealth regulations.
- Leverage mHealth for emerging markets.This may enable collaboration among med tech companies and other organizations to develop innovative solutions that can address patient access challenges while helping improve health outcomes in a cost-effective manner.