Understanding COVID-19 Diagnostic Tests
As efforts to increase diagnostic testing for COVID-19 accelerate, some consumers may still have questions about the types of tests available, who can perform them and where, and what differentiates one test from another. The guide below, adapted from the FDA website, should help answer some of these common questions.
COVID-19 Diagnostic Tests
In certain types of emergencies, the FDA can issue an emergency use authorization, or EUA, to provide more timely access to critical medical products (such as tests) that may help during the emergency when there are no adequate, approved, and available alternative options. The FDA has authorized for emergency use many tests that can diagnose infection with the virus that causes COVID-19, severe acute respiratory syndrome coronavirus 2 or SARSCoV-2.
Emergency Use Authorization
The EUA process is different than FDA approval, clearance, or licensing because the EUA standard is more flexible than the full approval, clearance, or licensing standard. Under an EUA, the data must show that a product may be effective and that the known a nd potential benefits outweigh the known and potential risks. This enables the FDA to authorize the emergency use of medical products that meet the criteria within days or weeks rather than months to years. The FDA has prioritized review of EUA requests for tests where authorization would increase testing accessibility or would significantly increase testing capacity. As a result, the FDA prioritizes review of EUA requests for point-of-care (POC) tests; home collection tests; at-home tests (none of which have been authorized as of October 2020); tests that reduce reliance on test supplies; and high-throughput, widely distributed tests.
See COVID-19 Solutions for Work and Schools→
Test performance
No test is 100% accurate, and test performance can vary based on the prevalence of disease in the population being tested. COVID-19 diagnostic tests may be less accurate in populations with a low prevalence of disease and in asymptomatic individuals, individuals who shed little virus, or individuals who are early or late in the course of illness. Tests are assessed based on their sensitivity and specificity. A test’s sensitivity is the fraction of positive cases that the test correctly identifies as positive, and a test’s specificity is the fraction of negative cases that the test correctly identifies as negative.
- A highly sensitive test will generally have a low false negative rate but will run a risk of false positives if the test’s specificity is low.
- A highly specific test will generally have a low false positive rate but will run a risk of false negatives if the test’s sensitivity is low.
To help mitigate the impact of false results, all COVID-19 tests authorized to date are prescription-only, so that clinicians can interpret results for patients.
Molecular versus antigen tests
Currently authorized SARS-CoV-2 diagnostic tests operate using one of two different underlying technological principles. These two diagnostic test types are molecular tests and antigen tests. Each type of test detects a different part of the SARS-CoV-2 virus particle.
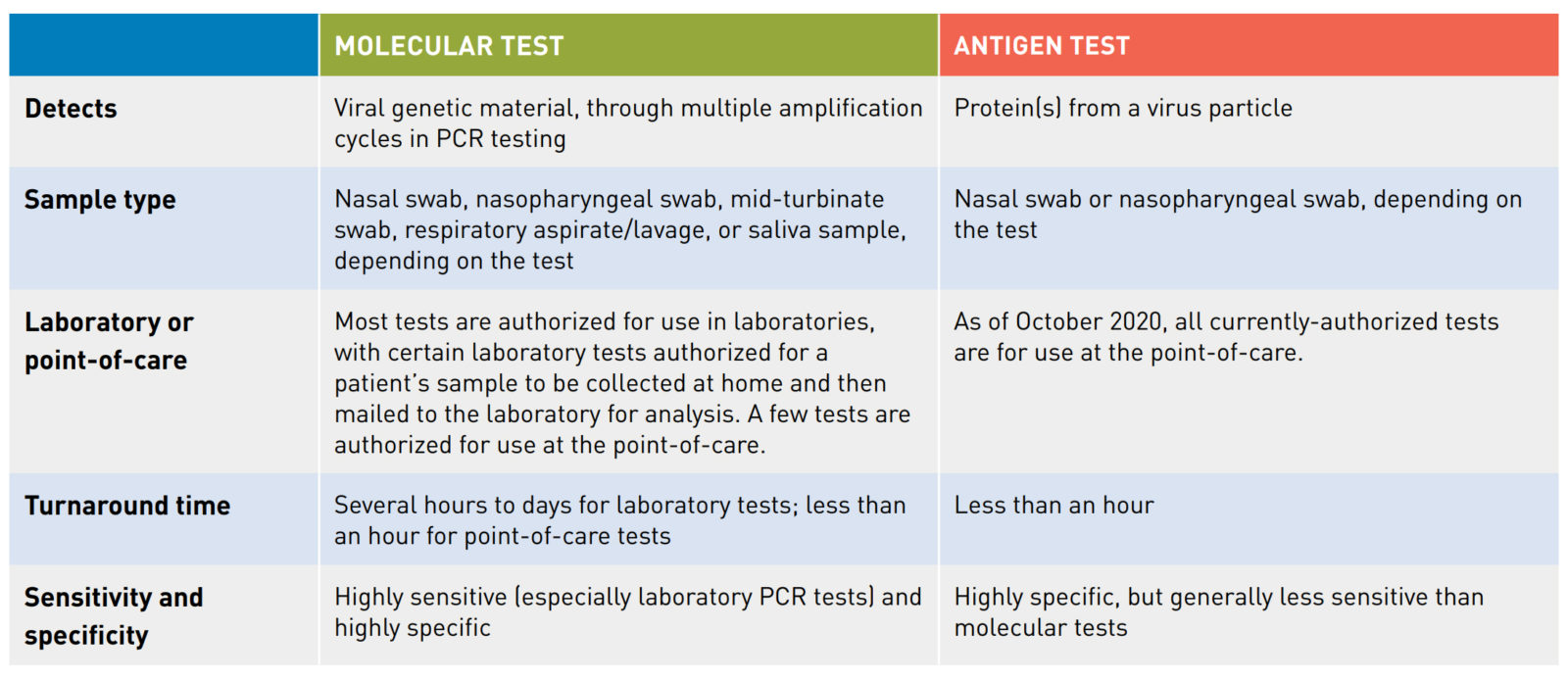
1. Molecular tests detect the genetic material or nucleic acid present inside a virus particle. The FDA has authorized molecular tests for use in a clinical laboratory setting and authorized some for use in a point-of-care (POC) setting. Most molecular tests are polymerase chain reaction (PCR) tests, also called nucleic acid amplification tests (NAAT). In PCR testing, a machine located in a laboratory or at a POC, depending on the test, runs a series of reactions. These reactions first convert the virus’s ribonucleic acid (RNA), if present, into deoxyribonucleic acid (DNA) and then amplify it (make millions of copies of the DNA); the test then detects this DNA. By running multiple amplification cycles, a PCR test can sense even low levels of viral genetic material in a patient’s sample, so these tests tend to be highly sensitive (especially laboratory PCR tests).
- A PCR test can be authorized to run batched or “pooled” patient samples if the developer demonstrates that the test meets the EUA standard for pooled testing. If a pooled sample tests positive, the samples that were combined then need to be tested individually to identify the positive case(s). When there is a low prevalence of cases (and a high number of negative results is therefore expected), pooling samples may result in fewer tests needing to be run and fewer testing supplies being required.
2. An antigen test detects one or more specific proteins from a virus particle. All currently-authorized antigen tests are POC tests and provide results in less than an hour. Antigen tests tend to be highly specific but are typically less sensitive than molecular tests. However, because antigen tests can generally be produced at a lower cost than molecular tests and have a simpler design, antigen tests could scale to test millions of individuals per day.
See COVID-19 Solutions for Work and Schools→
Conditions for use
When authorizing a product for emergency use, the FDA issues a Letter of Authorization. Each Letter of Authorization issued by FDA for a COVID-19 product is available on the agency’s website. These letters set forth the conditions of authorization, including the setting for conducting the test, the indications for using the test, and the responsibilities of the test’s distributors and users.
Laboratory versus point-of-care tests
The vast majority of SARS-CoV-2 diagnostic tests with EUAs are authorized for use in laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 41 U.S.C. §263a, to perform high or moderate complexity tests. Laboratory tests include some tests for which an individual’s nasal swab or saliva sample can be self-collected at home and then mailed to an authorized laboratory for analysis. However, as of October 2020, the FDA has not issued an EUA for any test for which the patient’s sample can be collected and tested for the virus at home. Laboratory tests are authorized either for the specific laboratory in which the test was developed or for any laboratory that meets certain requirements set forth in the EUA.
Several SARS-CoV-2 diagnostic tests are authorized to be conducted entirely at the point-of-care (POC) without a sample being sent to a laboratory for analysis. The term “point-of- care” refers to a patient care setting, such as any of the following that meets certain requirements:
- Doctors’ offices
- Nursing homes
- Urgent care centers
- Pharmacies
- School nurse offices
- Workplace health clinics
For POC tests, each test authorized to date has been authorized for use in patient care settings operating with a Certificate of Waiver, a Certificate of Compliance, or a Certificate of Accreditation under CLIA. The Centers for Medicare & Medicaid Services (CMS) issues CLIA Certificates and enforces compliance with CLIA regulatory requirements.
Worksite Testing Programs
As worksites face a surge of Coronavirus cases, many are turning to large-scale, onsite testing and vaccination programs to try and prevent the spread of the virus. The planning and administration of an employer-based testing program requires specialized expertise that Healthcare IT Leaders can provide. We offer PCR and Antigen testing and can manage the logistics, testing and reporting required for safety and regulatory compliance. If you have questions about testing protocols, pricing, or any other aspect of a worksite testing program, contact us today.
